research use only
Fluvastatin Sodium HMG-CoA Reductase inhibitor
Cat.No.S1909
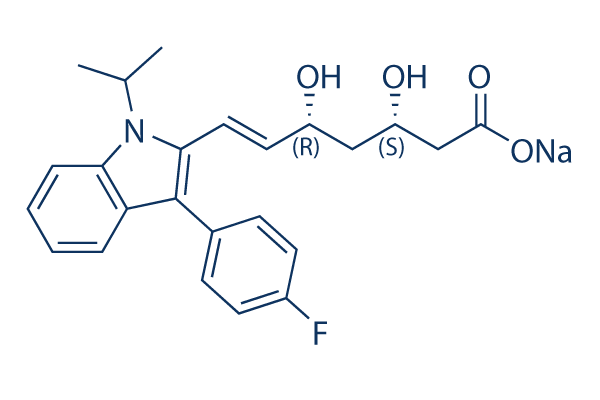
Chemical Structure
Molecular Weight: 433.45
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other HMG-CoA Reductase Inhibitors | Mevastatin SR-12813 Clinofibrate Dihydrolanosterol 7-ketocholesterol Cerivastatin sodium |
Chemical Information, Storage & Stability
| Molecular Weight | 433.45 | Formula | C24H25FNNaO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 93957-55-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | XU-62-320 Sodium | Smiles | CC(C)N1C2=CC=CC=C2C(=C1C=CC(CC(CC(=O)[O-])O)O)C3=CC=C(C=C3)F.[Na+] | ||
Solubility
|
In vitro |
DMSO
: 87 mg/mL
(200.71 mM)
Water : 18 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Features |
The order of magnitude of inhibition of each drug on the peroxidation was butylated hydroxytoluene > fluvastatin ≥ probucol ≥ pravastatin.
|
|---|---|
| Targets/IC50/Ki |
HMG-CoA reductase
(Cell-free assay) 8 nM
|
| In vitro |
Fluvastatin markedly inhibits the formation of thiobarbituric acid reactive substances in iron (II)-supported peroxidation of liposomes with IC50 of 12 μM. Fluvastatin ranging from 1 μM to 100 μM inhibits peroxyl radical-mediated peroxidation of liposomes induced by water-soluble and lipid-soluble radical generators, 2,2'-azobis (2-amidinopropane) dihydro-chloride and 2,2'-azobis (2,4-dimethylvaleronitrile), respectively. Fluvastatin (4 mM) and its metabolites shows superoxide anion scavenging activity in the hypoxanthine-xanthine oxidase system and a strong scavenging effect on the hydroxyl radical produced from Fenton's reaction. Fluvastatin (8 μM) and its metabolites shows protective effects on DNA damage as potent as the reference antioxidants, ascorbic acid, trolox, and probucol in CHL/IU cells. Fluvastatin (100 nM) shows a dose-dependent decrease in Ang II-activated superoxide anion formation in human aortic smooth muscle cells (hASMC). |
| In vivo |
Fluvastatin (10 mg/kg/day) results in a decrease in serum lipids in rabbits feed a 1.5% cholesterol containing diet. Fluvastatin (10 mg/kg/day) significantly lowers the tissue ACE in the aortae in rabbits feed a 1.5% cholesterol containing diet. Fluvastatin (10 mg/kg/day) significantly reverses the suppression of ACh-induced relaxation in rabbits feed a 1.5% cholesterol containing diet. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Growth inhibition assay | Cell viability |
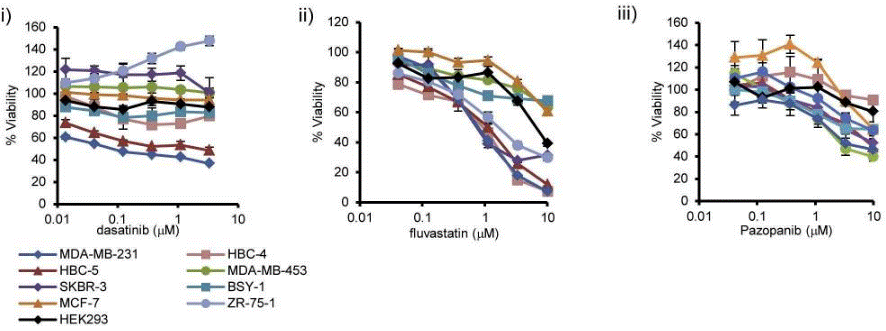
|
26199863 |
| Western blot | p53 / p21 / Cyclin D1 / ATF3 / H3P / PARP / Cleaved PARP / γ-H2AX YAP1 / TAZ / RHAMM |
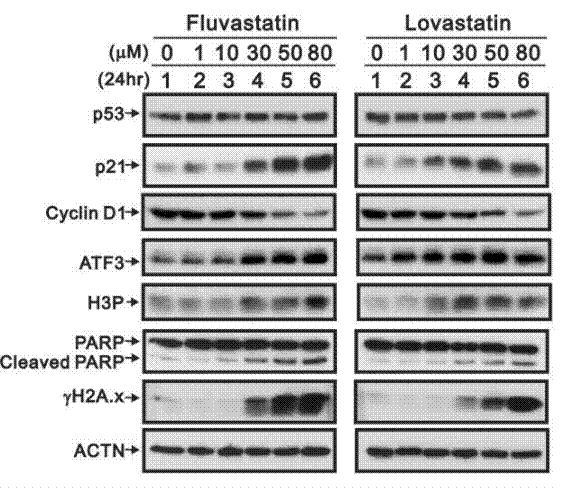
|
30939155 |
| Immunofluorescence | YAP1 |
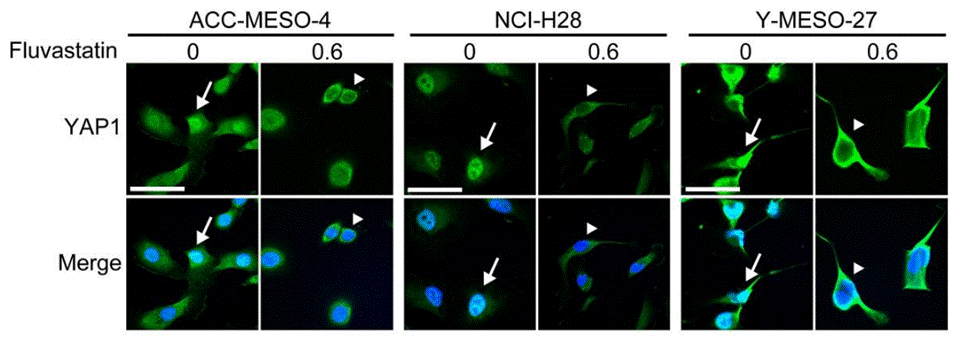
|
29212185 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02115074 | Completed | Glioma |
Centre Oscar Lambret|Anticancer Fund Belgium |
June 2014 | Phase 1 |
| NCT01524601 | Completed | Disorder Related to Renal Transplantation|Hypercholesterolemia |
University of Oslo School of Pharmacy|Oslo University Hospital |
February 2012 | Phase 4 |
| NCT00814606 | Withdrawn | Hepatitis C|Hepatitis C Virus |
University of Chicago |
February 2010 | Phase 2 |
| NCT00752843 | Completed | Healthy Subjects |
Corcept Therapeutics |
September 2008 | Phase 1 |
| NCT00674297 | Completed | Antiphospholipid Syndrome |
Hospital for Special Surgery New York|University of Texas |
May 2008 | Phase 2 |
| NCT00404287 | Terminated | Aortic Valve Stenosis |
AORTICA Group |
October 1 2006 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
