research use only
Gemfibrozil PPAR activator
Cat.No.S1729
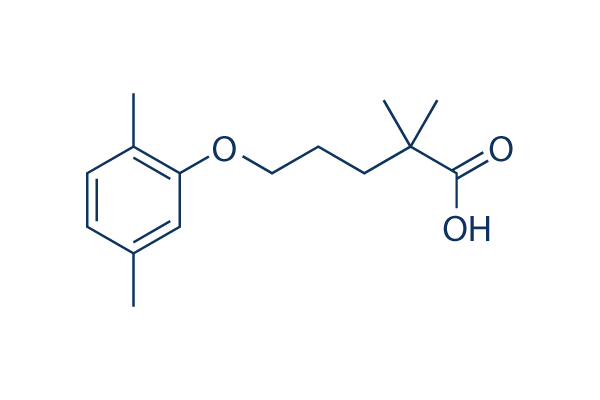
Chemical Structure
Molecular Weight: 250.33
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase Vitamin Carbohydrate Metabolism Mitochondrial Metabolism Drug Metabolite |
|---|---|
| Other PPAR Inhibitors | T0070907 GW9662 GW6471 WY-14643 (Pirinixic Acid) GSK3787 GW0742 AZ6102 Harmine Astaxanthin Eupatilin |
Chemical Information, Storage & Stability
| Molecular Weight | 250.33 | Formula | C15H22O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 25812-30-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CI-719 | Smiles | CC1=CC(=C(C=C1)C)OCCCC(C)(C)C(=O)O | ||
Solubility
|
In vitro |
DMSO
: 50 mg/mL
(199.73 mM)
Ethanol : 50 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
PPARα
|
|---|---|
| In vitro |
Gemfibrozil exerts a minimal inhibitory effect on CYP3A-mediated simvastatin hydroxy acid (SVA) oxidation, but does inhibit SVA glucuronidation in dog and human liver microsomes. This compound markedly inhibits M-23 formation, with a K(i) (IC(50)) value of 69 (95) mM, whereas inhibition of M-1 formation is weaker with a K(i) (IC(50)) value of 273 mM in human liver microsomes. It strongly and competitively inhibits CYP2C9 activity, with a K(i) (IC(50)) value of 5.8 (9.6) mM. This chemical exhibits somewhat smaller inhibitory effects on CYP2C19 and CYP1A2 activities, with K(i) (IC(50)) values of 24 (47) mM and 82 (136) mM, respectively. This compound, a lipid-lowering drug, inhibits cytokine-induced production of NO and the expression of inducible nitric-oxide synthase (iNOS) in human U373MG astroglial cells and primary astrocytes. It induces peroxisome proliferator-responsive element (PPRE)-dependent luciferase activity, which is inhibited by the expression of DeltahPPAR-alpha, the dominant-negative mutant of human PPAR-alpha. This chemical strongly inhibits the activation of NF-kappaB, AP-1, and C/EBPbeta but not that of gamma-activation site (GAS) in cytokine-stimulated astroglial cells.
|
| In vivo |
Gemfibrozil treatment significantly reduces (2-3-fold) the plasma clearance of SVA and the biliary excretion of SVA glucuronide (together with its cyclization product SV), but not the excretion of a major oxidative Metabolite of SVA in dogs.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06064539 | Completed | Multiple Sclerosis |
Sanofi |
May 18 2020 | Phase 1 |
| NCT03832595 | Completed | Chronic Kidney Diseases |
University of Pittsburgh|Vanderbilt University Medical Center|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) |
May 1 2019 | Not Applicable |
| NCT01736254 | Completed | Healthy Volunteers |
Eli Lilly and Company |
December 2012 | Phase 1 |
| NCT01797198 | Completed | Drug-Drug Interaction (DDI)|Healthy Subjects |
Astellas Pharma Europe B.V.|Astellas Pharma Inc |
April 2012 | Phase 1 |
| NCT01913379 | Completed | Healthy Subjects|Pharmacokinetics|Drug-Drug Interaction |
Astellas Pharma Europe B.V.|Medivation Inc.|Astellas Pharma Inc |
August 2011 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
