research use only
Mycophenolic acid Antineoplastic and Immunosuppressive Antibiotics inhibitor
Cat.No.S2487
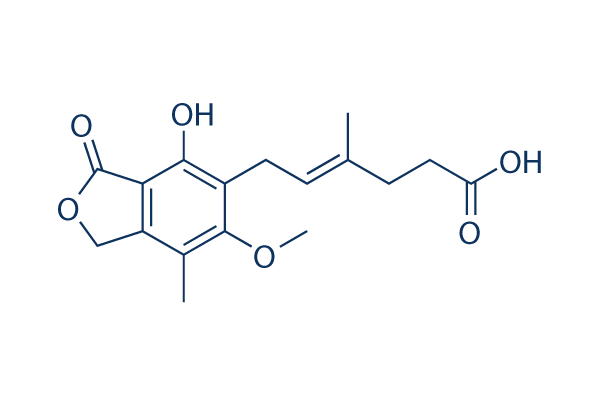
Chemical Structure
Molecular Weight: 320.34
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Antineoplastic and Immunosuppressive Antibiotics Inhibitors | Staurosporine (STS) Cyclosporin A Oligomycin A (MCH 32) Puromycin Dihydrochloride Nigericin sodium salt Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Sodium Monensin (NSC 343257) Cephalomannine |
Chemical Information, Storage & Stability
| Molecular Weight | 320.34 | Formula | C17H20O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 24280-93-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Mycophenolate, RS-61443 | Smiles | CC1=C2COC(=O)C2=C(C(=C1OC)CC=C(C)CCC(=O)O)O | ||
Solubility
|
In vitro |
DMSO
: 64 mg/mL
(199.78 mM)
Ethanol : 32 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
IMPDH
|
|---|---|
| In vitro |
Mycophenolic acid inhibits inosine-5'-monophosphate Dehydrogenase (IMPDH) by acting as a replacement for the nicotinamide portion of the nicotinamide adenine dinucleotide cofactor and a catalytic water molecule. This compound, an inhibitor of inosine monophosphate dehydrogenase, in nanomolar concentrations blocks proliferative responses of cultured human, mouse and rat T lymphocytes and B lymphocytes to mitogens or in mixed lymphocyte reactions. It suppresses mixed lymphocyte reactions when added 3 days after their initiation. This agent also inhibits antibody formation by polyclonally activated human B lymphocytes. It is an immunosuppressive agent reversibly inhibiting proliferation of T and B lymphocytes and antibody formation, with a profile of activity different from that of other immunosuppressive drugs. This chemical inhibits the formation of antibodies to sheep erythrocytes. It also suppresses, in a dose-related manner, the generation of cytotoxic T-lymphocytes against allogeneic cells in mice and prevents the elimination of allogeneic cells. This compound induces apoptosis and cell death in a large proportion of activated CD4+ T cells.
|
| In vivo |
Mycophenolic acid has lymphocyte-selective anti-proliferative effects in mice and can inhibit both cell-mediated and humoral immune responses without major side effects.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05702931 | Not yet recruiting | Hyperglycemia|Renal Transplant Complication Primary Non-Function|Diabetes |
Rigshospitalet Denmark|Aarhus University Hospital|Odense University Hospital |
April 1 2024 | Phase 4 |
| NCT06268769 | Recruiting | Immunosuppression |
Edward Geissler|Chiesi Pharmaceuticals GmbH|University of Regensburg |
March 9 2024 | Phase 4 |
| NCT06001320 | Recruiting | Kidney Transplant; Complications|CMV |
Virginia Commonwealth University|Merck Sharp & Dohme LLC |
September 25 2023 | Early Phase 1 |
| NCT05859191 | Recruiting | Systemic Lupus Erythematosus|Physiopathology |
University Hospital Tours|Research Center for Respiratory Diseases Inserm U1100 |
July 21 2023 | -- |
| NCT06361732 | Recruiting | Pediatric Liver Transplant |
Institute of Liver and Biliary Sciences India |
December 17 2022 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
