research use only
TAS-102 (Trifluridine/Tipiracil Hydrochloride Mixture) Nucleoside Antitumour Agent
Cat.No.S8539
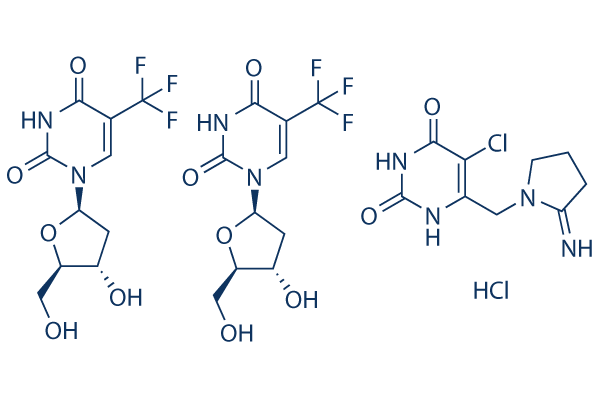
Chemical Structure
Molecular Weight: 435.76
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Other Thymidylate Synthase Inhibitors | Raltitrexed |
Chemical Information, Storage & Stability
| Molecular Weight | 435.76 | Formula | C10H11F3N2O5.0.5C9H11ClN4O2.0.5HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 733030-01-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Trifluridine-Tipiracil Hydrochloride Mixture | Smiles | Cl.OCC1OC(CC1O)N2C=C(C(=O)NC2=O)C(F)(F)F.OCC3OC(CC3O)N4C=C(C(=O)NC4=O)C(F)(F)F.ClC5=C(CN6CCCC6=N)NC(=O)NC5=O | ||
Solubility
|
In vitro |
DMSO
: 87 mg/mL
(199.65 mM)
Water : 29 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| In vitro |
TAS-102 is an oral combination drug consisting of trifluridine (FTD), which is a thymidine-based nucleoside analog, and tipiracil hydrochloride (TPI), which improves the bioavailability of FTD by inhibiting its catabolism by thymidine phosphorylase (TP). Phosphorylated form of trifluridine is incorporated into DNA resulting in DNA dysfunction and cell cycle arrest. Thymidine phosphorylase inhibitor inhibits degradation of FTD and inhibits angiogenesis. Thus, this compound treatment results in massive trifluridine incorporation into DNA and in activation of similar DNA damage response pathways, which involve phosphorylation of Chk1 and cycle arrest during the G2/M-phase. |
|---|---|
| In vivo |
The elimination half-life of FTD after intravenous administration to humans is very rapid (18 minutes), due to the rapid degradation of FTD to its major metabolite, 5-trifluoromethyl-2,4(1H,3H)-pyrimidinedione. In monkeys, the plasma FTD level after oral administration alone is very low, suggesting extensive first-pass metabolism by the liver and intestine TPase. However, the addition of TPI(tipiracil hydrochloride) is found to enable oral administration. By inhibiting TP, TPI inhibits the degradation of FTD in the liver and intestines following oral administration and thereby improves its bioavailability. The TP enzyme catalyses the phosphorolysis of pyrimidine 2'-deoxynucleosides such as FTD. Studies using human CRC tumour xenografts in mice determine that the maximum antitumour activity is achieved with a 1:0.5 molar ratio, and studies in mice and monkeys show that the maximum plasma concentration of FTD is almost achieved with the same ratio. Moreover, this ratio produces a favourable balance between antitumour activity and toxicity. Lower toxicity in mice is observed with TPI coadministration than with FTD alone. This compound can overcome acquired resistance to 5-FU because the main mechanism of this chemical is not associated with main metabolic enzymes of 5-FU, such as TS and OPRT. It has demonstrated efficacy in 5-FU-refractory cancers. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06039202 | Not yet recruiting | Metastatic Colorectal Cancer |
Holy Stone Healthcare Co. Ltd |
January 2024 | Phase 2 |
| NCT05343013 | Recruiting | Colorectal Cancer |
M.D. Anderson Cancer Center|Taiho |
June 6 2022 | Phase 2 |
| NCT04868773 | Active not recruiting | Colorectal Cancer|Colorectal Carcinoma|Metastatic Cancer|CRC |
University of California Irvine |
July 16 2021 | Phase 1 |
| NCT04074343 | Completed | Gastric Adenocarcinoma|GastroEsophageal Cancer |
University of California Irvine|Taiho Pharmaceutical Co. Ltd. |
August 26 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
