research use only
Zoledronic acid monohydrate Ras inhibitor
Cat.No.S5244
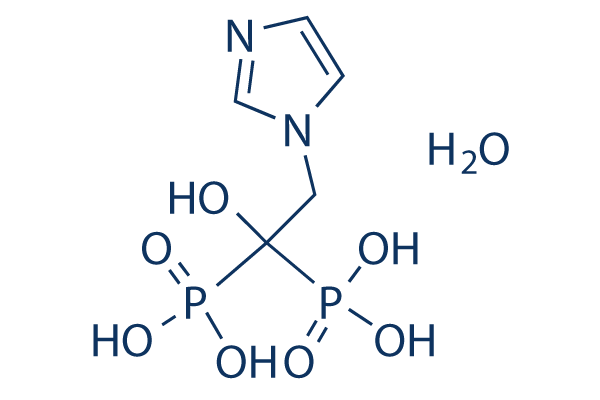
Chemical Structure
Molecular Weight: 290.1
Quality Control
| Related Targets | CDK HSP PD-1/PD-L1 ROCK Wee1 DNA/RNA Synthesis Microtubule Associated KRas Aurora Kinase Casein Kinase |
|---|---|
| Other Ras Inhibitors | ERAS-0015 RMC5127 Salirasib RBC8 Kobe0065 BQU57 CID-1067700 (ML282) ADT-007 MCP110 Perillyl alcohol |
Chemical Information, Storage & Stability
| Molecular Weight | 290.1 | Formula | C5H10N2O7P2.H2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 165800-06-6 | -- | Storage of Stock Solutions |
|
|
| Synonyms | zoledronate monohydrate, CGP-4244 monohydrate | Smiles | C1=CN(C=N1)CC(O)(P(=O)(O)O)P(=O)(O)O.O | ||
Solubility
|
In vitro |
0.5MNAOH : 6.02 mg/mL Water : 2.3 mg/mL
DMSO
: 0.01 mg/mL
(0.03 mM)
|
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
Mechanism of Action
| Targets/IC50/Ki |
Ras
Rho
|
|---|---|
| In vitro |
Zoledronic acid inhibits osteoclast maturation indirectly by increasing OPG protein secretion and decreasing transmembrane RANKL expression in human osteoblasts. Treatment of primary human OB-like cells with the potent nitrogen-containing BP, zoledronic acid (ZOL), resulted in a downregulation of membrane-associated RANKL protein expression. In addition to direct effects on cells of the osteoclast lineage, zoledronic acid may inhibit bone resorption by reducing transmembrane RANKL expression and increasing OPG secretion in osteoclast (OB)-like cells. Zoledronic acid induces growth inhibition (IC50:10–50 μM) and apoptotic death of human pancreatic cancer cells. The proapoptotic effect was correlated to cleavage/activation of caspase-9 and poly(ADP)-ribose polymerase, but not of caspase-3. It interferes with growth and survival pathways downstream to p21Ras. Zoledronic acid is also a potent inhibitor of angiogenesis. In vitro, zoledronic acid inhibits proliferation of human endothelial cells stimulated with fetal calf serum, basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (IC50 values 4.1, 4.2, and 6.9 μM, respectively), and modulates endothelial cell adhesion and migration. In cultured aortic rings and in the chicken egg chorioallantoic membrane assay, zoledronic acid reduces vessel sprouting. ZOL also exerted a concentration-dependent, biphasic effect on the adhesion and migration of HUVEC in vitro. ZOL concentrations of 1 and 3 μM increased cell adhesion but inhibited it at 30 and 100 μM. Similarly, cell migration was stimulated by 0.3 to 10 μM ZOL, whereas 30 μM completely inhibited it. These findings suggest that ZOL could interfere with cytoskeletal function in endothelial cells.
|
| In vivo |
Zoledronic acid affects breast cancer metastasis to visceral organs as well as bone. When administered systemically to mice, zoledronic acid potently inhibits the angiogenesis induced by subcutaneous implants impregnated with bFGF [ED50, 3 μg/kg (7.5 nmol/kg) s.c.]. In mice transplanted with osteosarcoma (OS) cells, ZOL administration prevented osteolysis and significantly reduced the amount of OS-induced bone formation while has no effect on tumour burden at the primary site. ZOL failed to reduce lung metastasis and in some cases was associated with larger and more numerous metastatic lesions.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05080790 | Recruiting | Leiomyosarcoma |
Institut für Klinische Krebsforschung IKF GmbH at Krankenhaus Nordwest|EUSA Pharma Inc. |
November 15 2021 | Phase 2 |
| NCT04719481 | Unknown status | Postmenopausal Osteoporosis |
Peking University Third Hospital |
November 2021 | Phase 4 |
| NCT04719650 | Not yet recruiting | Postmenopausal Osteoporosis |
Peking University Third Hospital |
October 2021 | Phase 4 |
| NCT04034199 | Unknown status | Idiopathic Inflammatory Myopathies|Osteoporosis Osteopenia |
Kwong Wah Hospital|Tung Wah Group of Hospitals |
August 15 2019 | Phase 3 |
| NCT03862833 | Completed | Hematopoietic Stem Cell Transplantation |
Nantes University Hospital |
May 7 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
